|
Title: The amphibamiform Nanobamus macrorhinus from the early Permian of Texas Authors: B.M. Gee, R.R. Reisz Journal: Journal of Paleontology Link to paper and DOI: 10.1017/jpa.2019.72 The history of Nanobamus The holotype and only known specimen has only been known for a few decades, but this taxon's already accrued a fairly lengthy taxonomic history.
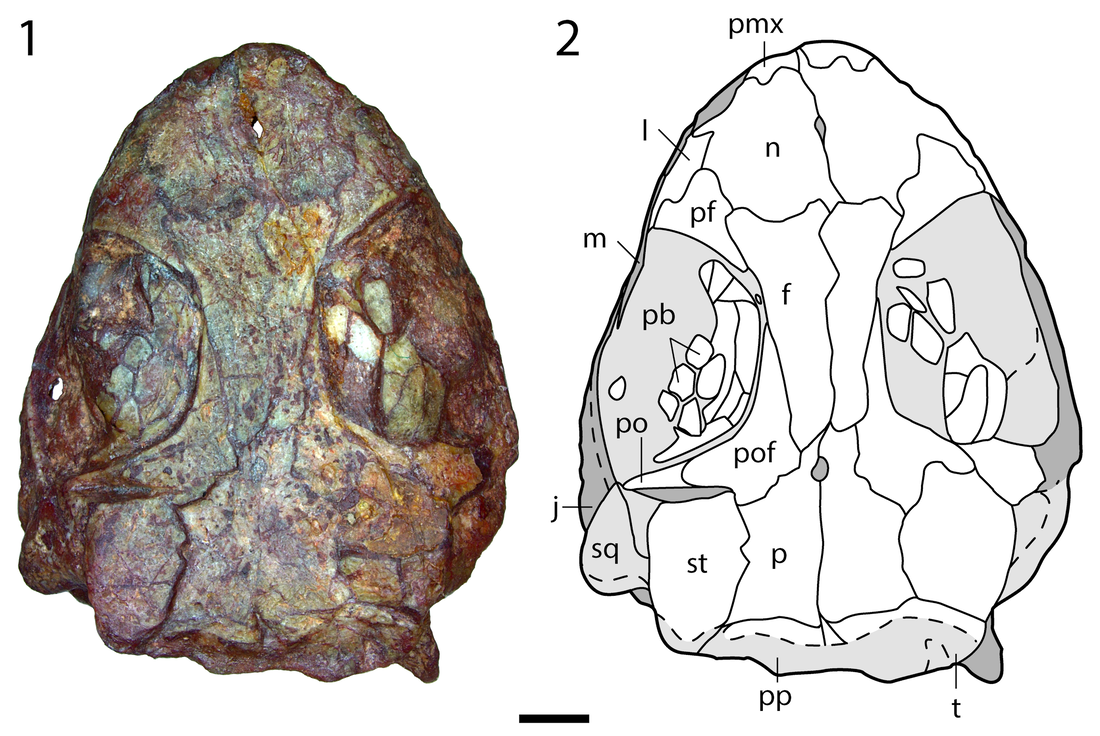
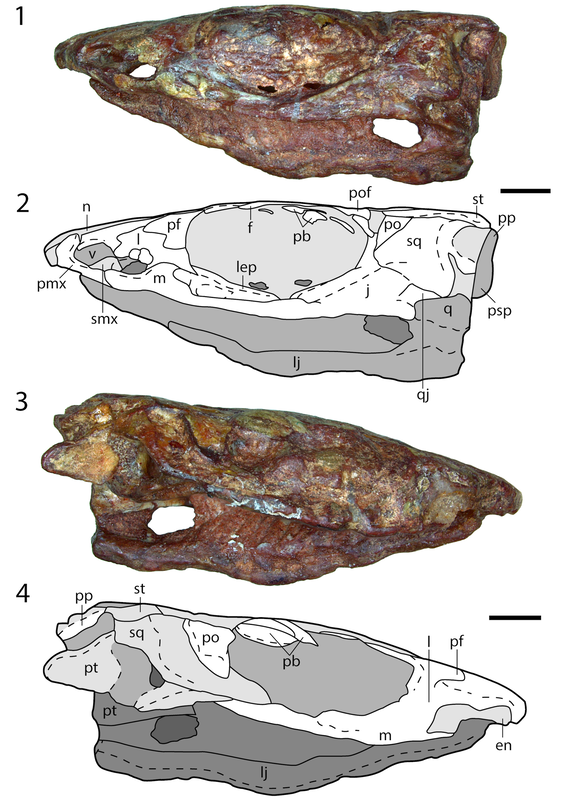
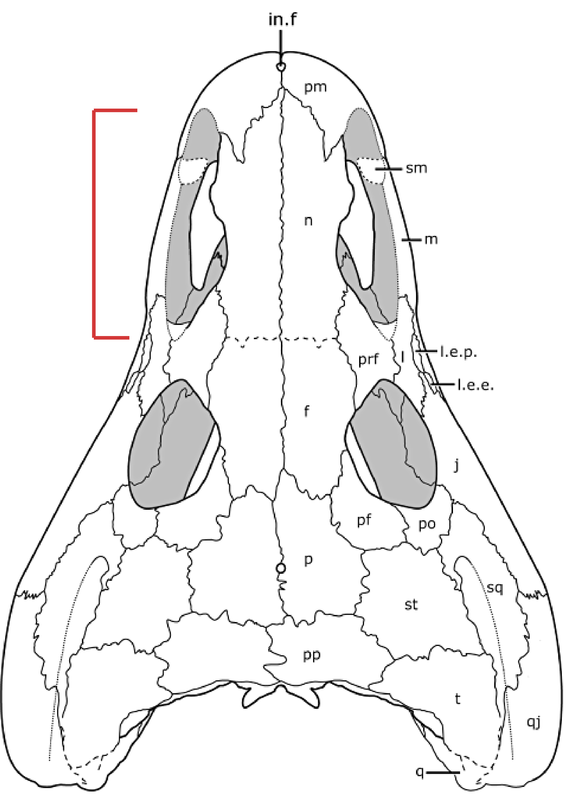
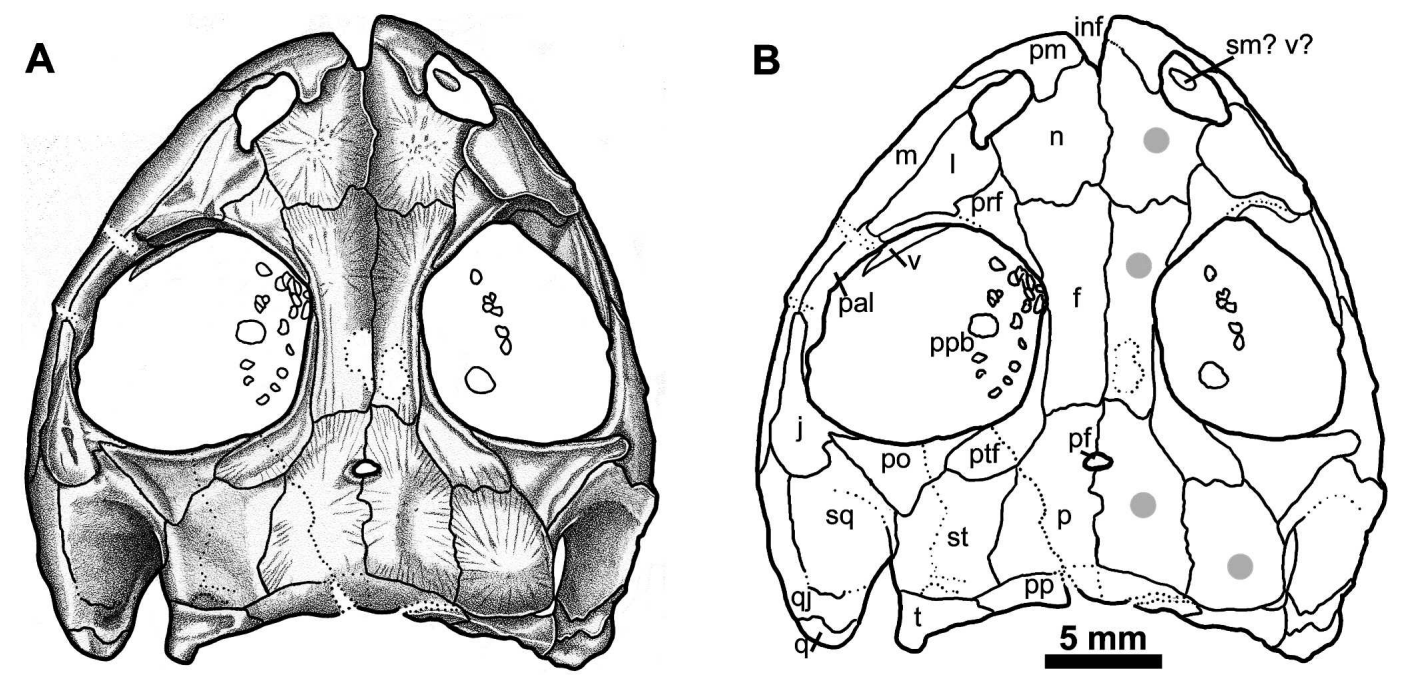
The peculiarities of long nostrils The classic poster child for weirdly long nostrils are trematopids, which often have a nostril that's at least as long as their eye (see Acheloma on the bottom right). Based on the position of the septomaxilla, a bone that sits within the external naris, we know that it wasn't just that their nasal organ got longer. So in other words, they probably didn't have a particularly acute sense of smell relative to other dissorophoids. However, it's not entirely clear what filled the back end of this long nostril. There have been a few suggestions (see Dilkes, 1993 for a summary), such as a salt gland, but there isn't much of a precedent for such a feature in modern amphibians that would provide a good analogue, and the inside of the nostril isn't particularly complex; most of the internal bony surface is smooth. Since all trematopids have a long nostril, it might relate to their generally large body size and predatory habits, but the independent appearance of a very similar nostril in the much smaller amphibamiforms is then even more peculiar. The nostril of Nanobamus is longer than that of Georgenthalia, but they both are formed by an incision into the lacrimal that still excludes the prefrontal from the naris (the prefrontal frames the naris dorsally in trematopids).
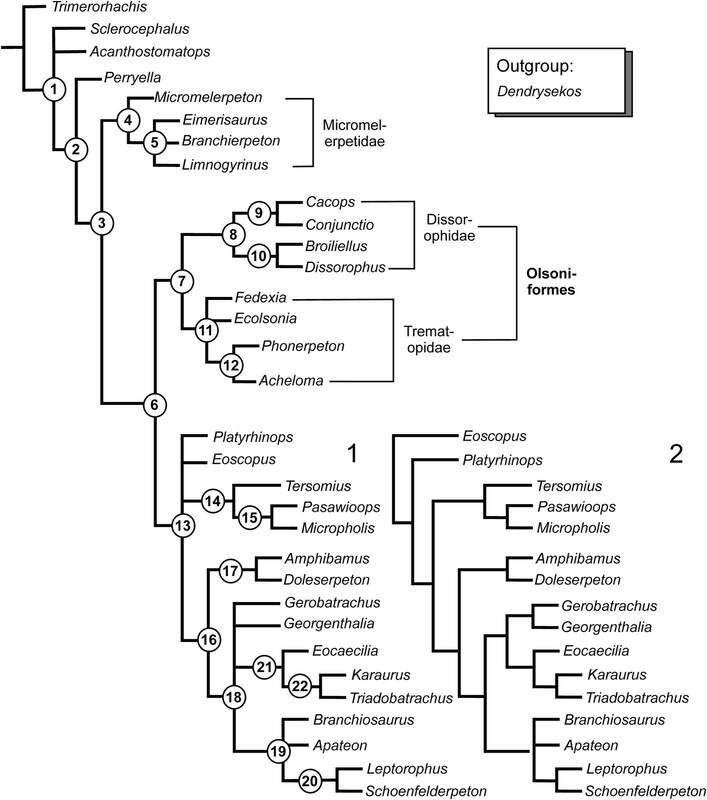
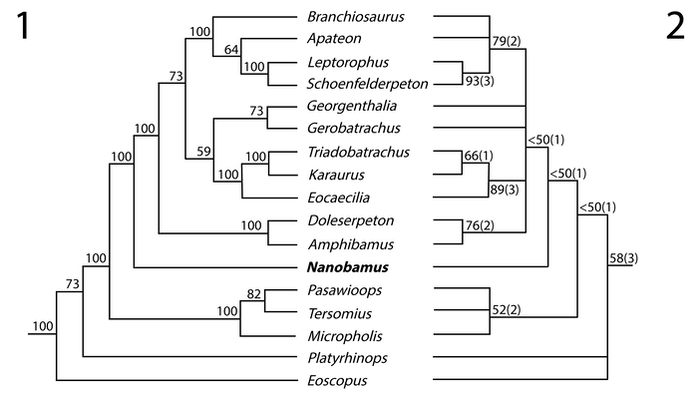
As you can see, the best supported nodes are those that aren't directly related to terrestrial amphibamiforms (Doleserpeton down through Eoscopus). The best nodes are nodes like crown Lissamphibia (Triadobatrachus, Karaurus, and Eocaecilia) or the ones for branchiosaurids (Branchiosaurus through Schoenfelderpeton). Virtually all of the terrestrial amphibamiforms are not well-supported beyond modest support for the newly defined Amphibamidae (Amphibamus + Doleserpeton) and Micropholidae (Pasawioops, Tersomius, Micropholis). What explains this? Amphibamiforms often have interesting mixtures of primitive and derived traits. Some of these might be convergent, even within amphibamiforms. For example, both Nanobamus and Georgenthalia have an elongate naris that is distinct from that of trematopids and essentially identical to each other, but they are not recovered as being closely related, which either indicates that the phylogeny is biased by some methodology (e.g., taxon sampling) or that this feature appeared twice in amphibamiforms, which would be quite interesting. The phylogeny also doesn't accord with the stratigraphic occurrence of taxa (not that it has to, but it is interesting to note). For example, Micropholis is one of the most primitive amphibamiforms, but it's the youngest one in the fossil record by a long shot (Early Triassic of South Africa). In contrast, Amphibamus is one of the most derived amphibamiforms, but it's one of the oldest (Carboniferous of North America). This can indicate many things, such as long ghost lineages with missing data from the fossil record, or problems with the existing character selection, or convergence that's being interpreted by the algorithms as the product of shared ancestry. All exciting things to keep exploring in the future! Refs
David Marjanović
9/11/2019 12:24:24 pm
*applause* Comments are closed.
|
About the blogA blog on all things temnospondyl written by someone who spends too much time thinking about them. Covers all aspects of temnospondyl paleobiology and ongoing research (not just mine). Categories
All
Archives
January 2024
|
 RSS Feed
RSS Feed
