|
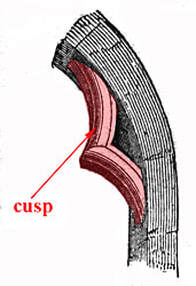
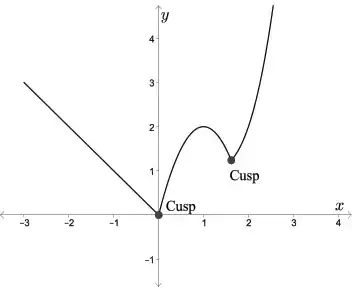
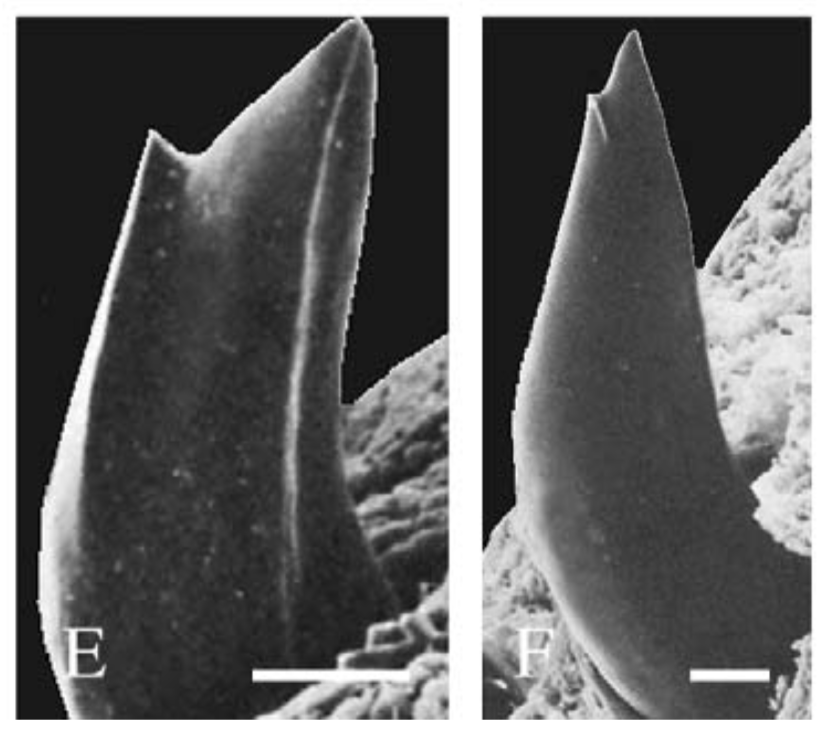
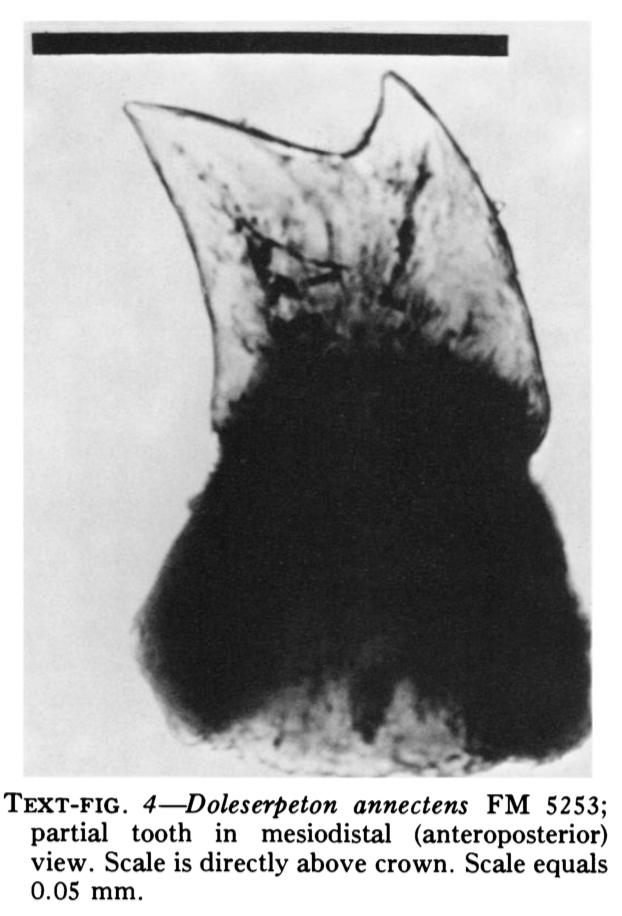
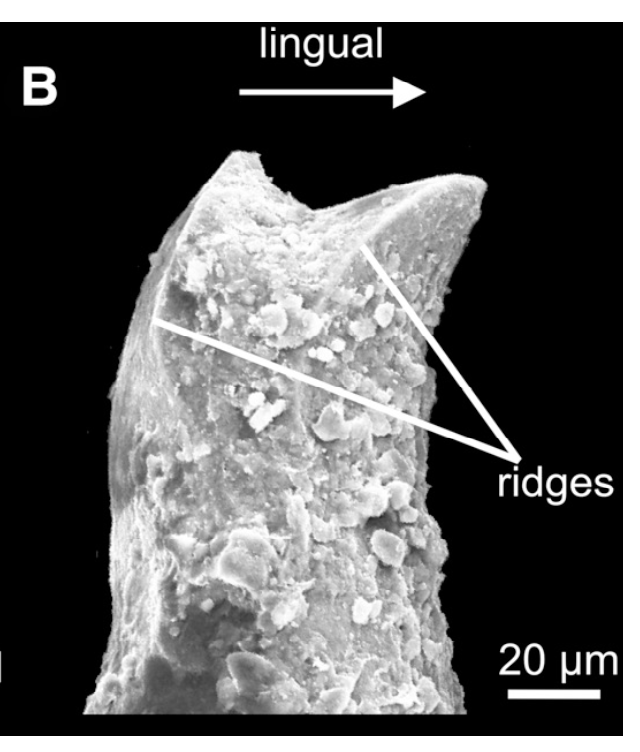
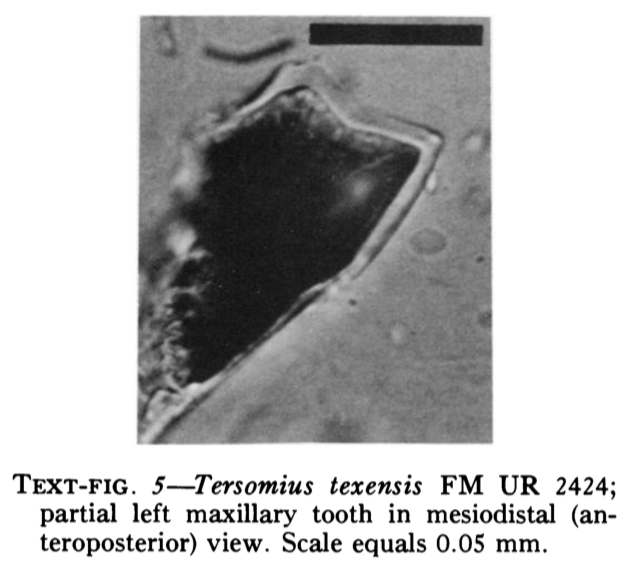
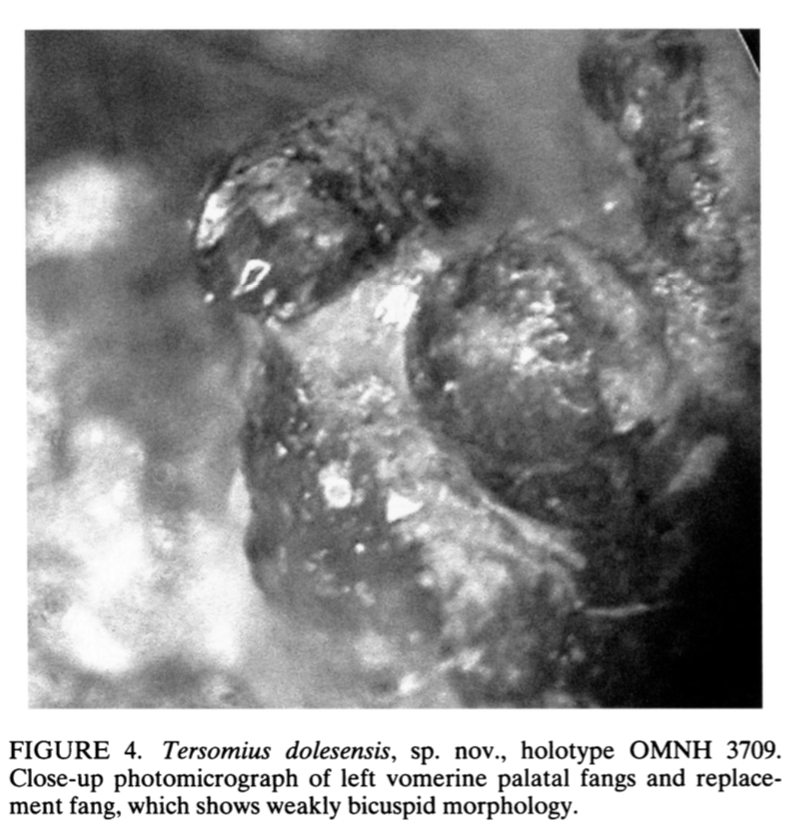
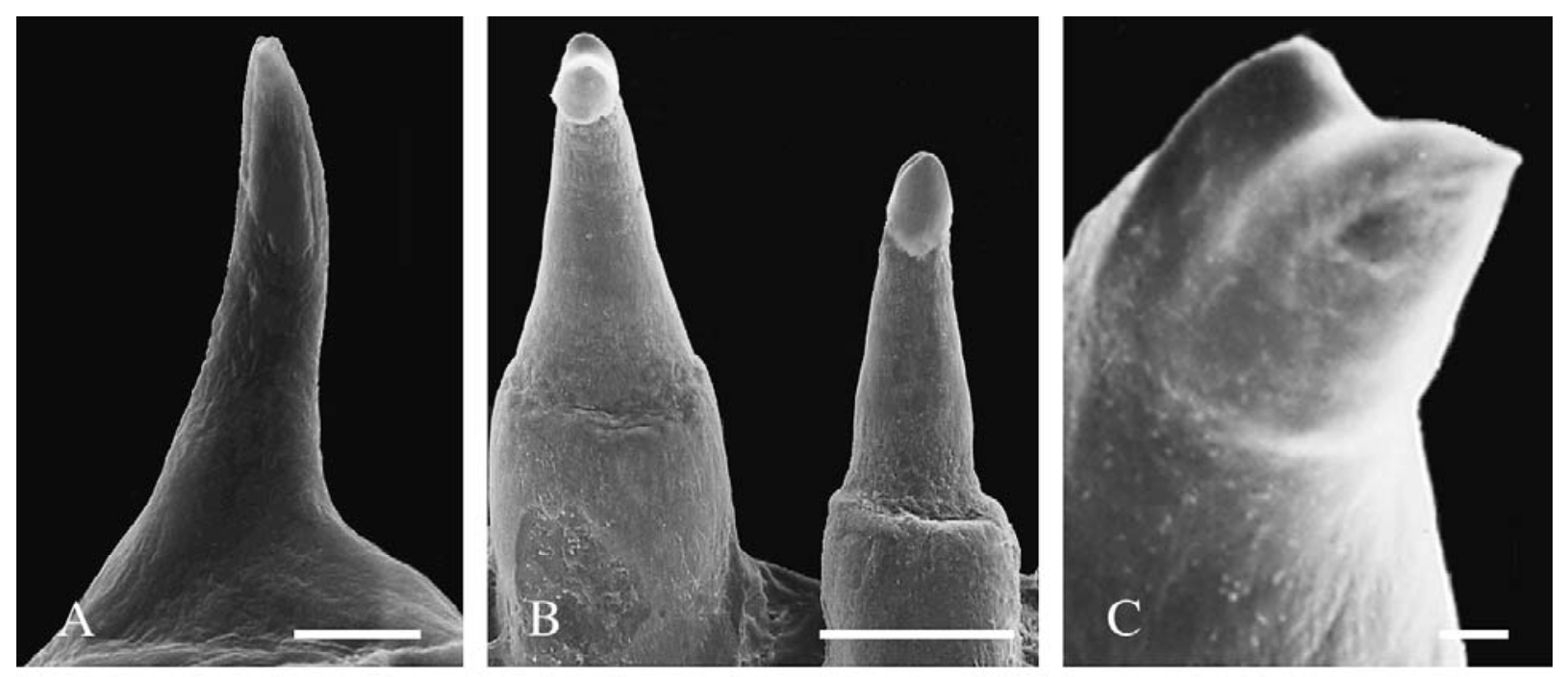
The average person is probably most accustomed to thinking of 'cusp' along the lines of "on the edge" or "on the verge." As in, "we're on the cusp of returning to a normal life with this vaccine." But 'cusp' actually has a different simple meaning, which is derived from the Latin word for 'point' or 'apex.' Architects use it to refer to a feature that became particularly popular in the Gothic period, a small projection between small arches. Mathematicians use it to refer to the point where a curve drastically changes directions. And biologists use it to refer to either a tissue fold that contributes to a valve to prevent blood backflow or to a pointed protrusion on the surface of the tooth. (There's also usage in astrology, but I'm not getting into that). I'm not an architect or a mathematician, nor do I work on blood vessels, so here we are talking about teeth! A decidedly non-model systemIn the old days, a lot of what we inferred about other "complex" animals was based on what we knew about humans, since we knew ourselves best (hypothetically), which is why a lot of anatomical features were first named based on humans. Of course, if you go to a nice nature area and look around, it's pretty easy to see that humans are not really a great "model" for other animals beyond primates. Ignoring things like complex societies and technology, we walk on two legs but can't fly, have reduced hair, and have opposable thumbs, features that are mostly shared (through common ancestry) only with other primates. Mammals are generally bad models for other animals, which is why when scientists refer to "model organisms" that are used for their combination of adaptability to lab settings and applicability to a wide range of biological systems, we don't use many mammals. Anyone who's been through a high school or college biology class probably knows a little bit about the humble common fruit fly (Drosophila melanogaster), or perhaps the microscopic nematode Caenorhabditis elegans (usually just called 'C elegans' to dodge the wordy genus name), or maybe even the quietly invasive African clawed frog (usually called Xenopus by scientists and not to be confused with the African dwarf frog that Petsmart sells). These are considered model organisms for many reasons, none the least of which is that they're very hardy in lab settings, but also because their anatomy/physiology makes them reasonable proxies for something that's more complex and harder (or impossible) to work with in labs. At least some readers of the blog may be surprised to know that in the 50's, the frog I mentioned was used as a living pregnancy test for women with shocking accuracy (details in article; frogs not really harmed in process). I'm pretty sure it would be illegal to do the test in reverse to test whether a frog was pregnant by experimenting on a human. Which is one reason why Xenopus continues to be a model organism despite being discontinued as a pregnancy test. Coincidentally, Xenopus is also a good model for the topic of today's blog post - the primitive tooth condition in tetrapods. Teeth come in a wide variety of shapes, none more evident than in our own mouth, in which the various tooth types are named as such because they look different. Most animals don't have this kind of differentiation though (called 'heterodonty'); we call animals without differentiated teeth 'homodont.' Look in the mouth of the fish on your cutting board, and you're likely to see a lot of teeth that all look the same. They probably look quite simple too - simple cones with a single point. We call this tooth 'monocuspid' because it has one cusp. Teeth with two cusps are 'bicuspid,' teeth with three cusps are 'tricuspid,' and so on (though usually 4+ is just called 'multicuspid'). Monocuspid teeth are found in Xenopus, as well as most early tetrapods, including most temnospondyls. But Xenopus is an outlier among its froggy friends. Most frogs actually have bicuspid teeth (although toads, which are frogs, have no teeth), and a handful are reported to have tricuspid teeth (e.g., Greven & Ritz, 2009), but none have more than three. If you want an example of a tooth with more than three cusps, just look at your own molars - there are 4-5 in humans. Bicuspid teeth are typical for salamanders and caecilians, the other two groups of living amphibians, but they are not super common in animals with homodont dentition or are only found in part of the mouth (our premolars are bicuspid, which you maybe heard your dentist say when looking at your x-rays). Amphibians usually have homodont dentition (same size and shape throughout the mouth), so naturally, this has put an emphasis on searching for bicuspid teeth in the fossil record as one means of elucidating the origins of modern amphibians. Tales of the teethLike I mentioned before, monocuspid teeth are the base model for tetrapods and temnospondyls. This isn't really surprising; adding points is complex and requires more nuanced spatial control over the tooth's development rather than just forming one point. There is only one example of a temnospondyl with tricuspid teeth, the branchiosaurid Tungussogyrinus from Siberia (Werneburg, 2009). The broad acquisition of more complex teeth, as well as heterodont dentition, tracks the expansion of tetrapods into different ecological roles, more specifically their dietary preferences. Scraping algae off of rocks, for example, is apparently a key driver of tricuspid teeth in marine iguanas (e.g., Melstrom, 2017); this idea has been extended down to some extinct tetrapods with tricuspid teeth as well (e.g., Mann & Maddin, 2019). Temnospondyls, like amphibians today, aren't picky, but they aren't generalists either. Amphibians can't really eat plant matter, for example, whereas the capacity for herbivory was an acquisition that facilitates the diversification of amniotes on land. But temnospondyls did eat other animals, and for that, monocuspid teeth work just fine, whether for grabbing, piercing, or stabbing. There are several examples of temnospondyls with bicuspid teeth, or to be a little safer, several taxa in which bicuspid teeth are reported. Probably the most unequivocal of them is Doleserpeton annectens, which John Bolt keyed in on in the 1960s as a "proto-lissamphibian" based on his observation of this feature. With a lot of material and excellent preservation, subsequent research has confirmed the presence of bicuspid teeth in this taxon (e.g., Sigurdsen & Bolt, 2010). With the recognition of this feature in one amphibamiform, workers began looking for more evidence of bicuspid teeth in what was then called 'amphibamids.' Amphibamus, a close relative of Doleserpeton, also has bicuspid teeth (Bolt, 1979). Platyrhinops, which is somewhat debated over whether it is the same as Amphibamus, also has bicuspid teeth (Clack & Milner, 2009). Most amphibamiforms do not have bicuspid teeth however, including Gerobatrachus, the 'frogamanader' ('batrachian' is the technical term for the grouping of frogs and salamanders; Anderson et al., 2008). There is also some controversy about whether any species of Tersomius has bicuspid teeth. Bolt (1977) indicated some specimens of Tersomius texensis (but not the holotype) have bicuspid teeth, but he only figured one of these bicuspid teeth (it was hard to get clear photographs or microphotographs in the 70's). The specimen that he did figure does appear to have two cusps, but they are not nearly as well-developed as in Doleserpeton or in most living amphibians. Probably important to bear in mind here is that we're talking about teeth that are less than one-tenth of a millimeter in width, so the precision in the plane of sectioning could be a factor. Notable too is that all of the Tersomius material with bicuspid teeth is from the South Grandfield site in Oklahoma; possibly, it is not actually T. texensis (or Tersomius at all). The original assignment was provisional (cf. designation by Daly, 1973) and made no comments on the teeth. That material has not been redescribed. This is further complicated by Anderson & Bolt's (2013) description of Tersomius dolesensis. Not only does it have the peculiar combination of monocuspid marginal teeth with at least one bicuspid palatal tooth, but there is disagreement about whether this is actually properly placed in Tersomius (their phylogenetic analysis excludes other species). In Maddin et al.'s (2013) analysis with the type and one good referred specimen of T. texensis, T. dolesensis does not group with what are now termed micropholids (Pasawioops + Micropholis + Tersomius). Suffice it to say that some amphibamiforms have bicuspid teeth and most do not. 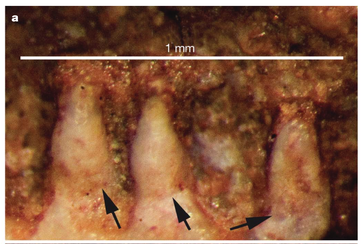 Monocuspid teeth of the amphibamiform Gerobatrachus (Anderson et al., 2008) - this is one of the closest relatives of at least frogs + salamanders under many hypotheses. Monocuspid teeth of the amphibamiform Gerobatrachus (Anderson et al., 2008) - this is one of the closest relatives of at least frogs + salamanders under many hypotheses. Unsurprisingly, paleontologists have looked to other possible lissamphibian candidates, primarily 'microsaurs,' to see whether they too might have bicuspid teeth (diminishing the value of bicuspidity in amphibamiforms). And indeed, there are numerous reports of bicuspidity among 'microsaurs,' including Carrolla craddocki (Langston & Olson, 1986; Maddin et al., 2011), the now defunct 'Bolterpeton carrolli' (Anderson & Reisz, 2003; Bolterpeton is synonymous with the parareptile Delorhynchus), and Cardiocephalus peabodyi (Anderson & Reisz). The description of two cusps in C. craddocki still stands, whereas that of C. peabodyi seems to have been quietly abandoned. Haridy et al. (2017) suggest that in taxa with cutting edges on the teeth, like in many parareptiles, these edges may be misinterpreted as the ridges leaving to two cusps; they specifically suggest that what used to be 'B. carrolli' appeared to have two cusps only because of uneven tooth wear. It's also worth noting that the putative stem caecilian Chinlestegophis, a stereospondyl temnospondyl, also lacks bicuspid teeth (Pardo et al., 2017), as does the more highly nested amphibamiform Gerobatrachus (Anderson et al., 2008). It's just a phaseOne of the key caveats in bicuspidity in modern amphibians is that it is known to change in the development of the animal. Teeth are monocuspid early in development and only later do they become bicuspid. This is one of the reasons why John Bolt proposed that some amphibamiforms might in fact be juvenile dissorophids, and he proposed the inverse scenario in which the adult stage was a monocuspid tooth that transitioned from a bicuspid tooth. Features that are transient throughout an animal's life become difficult to work with in the fossil record because it means that absence (of bicuspidity in this case) is not necessarily attributable to a legitimate absence of homologous features - instead, the material under study might be immature and not yet have achieved the derived condition that is being sought but would have at a more advanced stage of life. Bolt wasn't the first to suggest that some terrestrial amphibamiforms might actually be juveniles of larger animals (same problem with the mostly aquatic branchiosaurids), but the data collected since his work in the 70s and 80s has not supported this conjecture. A higher purpose
References
|
About the blogA blog on all things temnospondyl written by someone who spends too much time thinking about them. Covers all aspects of temnospondyl paleobiology and ongoing research (not just mine). Categories
All
Archives
January 2024
|




 RSS Feed
RSS Feed
